Concentrated H 2 SO 4. When you are said to write balanced equation remember that to write physical properties of compounds.

How To Balance Ca Hcl Cacl2 H2 Calcium Hydrochloric Acid Youtube
Sodium hydroxide and hydrochloric acid react to form sodium chloride and water.

. C 2 H 6 72 O 2 2CO 2 3H 2 O x 2. We will guide you on how to place your essay help proofreading and editing your draft fixing the grammar spelling or formatting of your paper easily and cheaply. Calcium and bicarbonate are two other electrolytes found in extracellular fluid.
I 4Nas O 2 g 2Na 2 Os ii CuO s H 2 g Cu s H 2 Ol Answer. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride. NaOH Caustic Soda H_2O Water.
The acidity produced due to excess hydrochloric acid in the stomach which cause indigestion produce pain and irritation. NaCl Sodium Chloride Salt Online chemical balancing calculator allows you to make a balanced chemical equation immediately. Calcium chloride is produced in commercial amounts using many procedures.
When hydrogen chloride gas dissolves in water a it reacts as an acid transferring protons to water molecules to yield b. It is found in. A polyprotic acid is an acid that has two or more hydrogen ions to donate per formula.
Get 247 customer support help when you place a homework help service order with us. There are 7 O-atoms on RHS. Since we only answer up to 3 sub-parts well answer the first 3.
Write the equation and balance it if necessary NaClaq AgNO 3 aq AgCls NaNO 3 aq Step 2. It is also known as hydrogen chloride or muriatic acid. Examples are hydrochloric acid HCl a strong acid and acetic acid HC2H302 a weak acid.
Please resubmit the question and. It is insoluble in ethanol and acetic acid but soluble in dilute nitric acid and hydrochloric acid. It is a strong corrosive acid with a chemical formula HCl.
Sodium chlorideaq silver nitrateaq silver chlorides sodium nitrateaq Solution. When HCl reacts with calcium carbonate calcium chloride and carbon dioxide gas are given. In Section 21 we have seen that all acids have similar chemical.
Definition of An Acid For example hydrochloric acid dissolves in water to form hydrogen ions and chloride ions. When something is stable it should have less energy. A property possessed by some substances of absorbing moisture from the air on exposure.
Milk of magnesia chemically magnesium hydroxide is used as an antacid. Calcium is needed to. 22 WHAT DO ALL ACIDS AND ALL BASES HAVE IN COMMON.
Write the ionic equation for the acid-carbonate reaction between hydrochloric acid and insoluble magnesium carbonate to form magnesium chloride salt water and carbon dioxide. The balanced equation for the reaction. An acid is a substance which produces hydrogen ions H aq in water.
Question 3 Identify the substances that are oxidised and the substances which are reduced in the following reactions. Calcium carbonate and hydrochloric acid balanced equation. Hydrochloric acid is nothing but a solution of hydrogen chloride mixed with water.
Refining of natural brines reaction of calcium hydroxide with ammonium chloride in Solvay soda ash production and reaction of hydrochloric acid with calcium carbonate. Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated multiplied by 100. This reaction involves the application of heat to calcium nitrate to decompose it into calcium oxide nitrogen i oxide and oxygen gas.
Yield actual yieldtheoretical yield 100 So lets say you want to do an experiment in the lab. 2HClaq MgCO 3 s MgCl 2 aq H 2 Ol CO 2 g. C 2 H 6 O 2 2CO 2 3H 2 O.
Write the ionic equation for the word equation. Another industrial method of preparing benzoic acid is by reacting tri-chlorotoluene with calcium hydroxide in the presence of water and the treatment of the calcium benzoate product with hydrochloric acid. Only compounds that are aqueous are split into ions.
In the 1980s there was an international agreement to de-stroy all stockpiles of mustard gas ClCH₂CH₂SCH₂CH₂ClWhen this substance contacts the moisture in eyes nasal pas-sages and skin the -OH groups of water replace the Cl atoms and create high local concentrations of hydrochloric acid which cause severe blistering and tissue destruction. Examples include sulfuric acid H2S04 a diprotic acid and phosphoric acid H3P04 a triprotic acid. Hydrochloric acid is considered to be the simplest acidic system that contains chlorine and water.
Thus after removing the. Our global writing staff includes experienced ENL ESL academic writers in a variety of disciplines. CaCO 3s 2HCl aq CaCl 2aq.
Calcium is the major cation involved in the structure and function of bones and teeth. Since it is basic in nature reacts with the excess hydrochloric acid present in the stomach and neutralises it. I Substances oxidised is Na as it gains oxygen and oxygen.
However an online Percent Yield Calculator helps you to calculate the percent yield value by adding theoretical yield and actual yield value. Aluminum and hydrochloric acid react to form aluminum. To make 7 O-atoms at LHS we have to write 72 before O 2 but we can use only whole number to balance the equation so we write 72before O 2 and multiply the whole equation by 2.
The structure of a C 6 H 5 COOH molecule is illustrated below. Metal compound A reacts with dilute hydrochloric acid to produce effervescence. The process represented by this equation confirms that hydrogen chloride is an acid.
This is a balanced chemical equation representing the reaction between sodium metal and hydrochloric acid reactants to produce sodium chloride solution and hydrogen gas products. Phosphorous pentoxide and calcium oxide are good drying agents but they cannot be used to dry hydrogen chloride gas because they react with hydrogen chloride. How can I balance this chemical equations.
A 00665 g sample of aluminum metal reacts with hydrochloric acid to give 905 mL of hydrogen gas at A. Now to balance the atoms in carbon multiply CO 2 molecules by 2. It slightly dissolves in water.
It is also known to be highly acidic and has the ability to attack and cause damage to the skin. The drying agent used in drying hydrogen chloride gas is conc. Calcium phosphate appears as a white amorphous or crystalline powder that is odourless and tasteless.
Gastric mucosal cells need chloride to produce hydrochloric acid which breaks down food into absorbable components. You want to measure how much water is produced when 120 g of glucose C_6H_12O_6 is burned with enough oxygen. To learn more about the Preparations Properties Uses and Videos with FAQs of.
Chloride helps maintaining osmotic pressure. This lets us find the most appropriate writer for any type of assignment. The gas evolved extinguishes a burning candle.
HClaq H aq Cl- aq It is the hydrogen ions which turn blue litmus to red and give acids their characteristic properties. When dissolved in water H 3 O ions are produced by a chemical reaction in which H ions are transferred from HCl molecules to H 2 O molecules. Hydrochloric acid is known to have a very pungent odour.
HCl Hydrochloric Acid. HCl Acid Hydrochloric Acid - Hydrochloric acid is an inorganic chemical.

How To Balance Ca Hcl Cacl2 H2 Calcium Hydrochloric Acid Youtube

How To Balance Caco3 Hcl Cacl2 Co2 H2o Calcium Carbonate Hydrochloric Acid Youtube
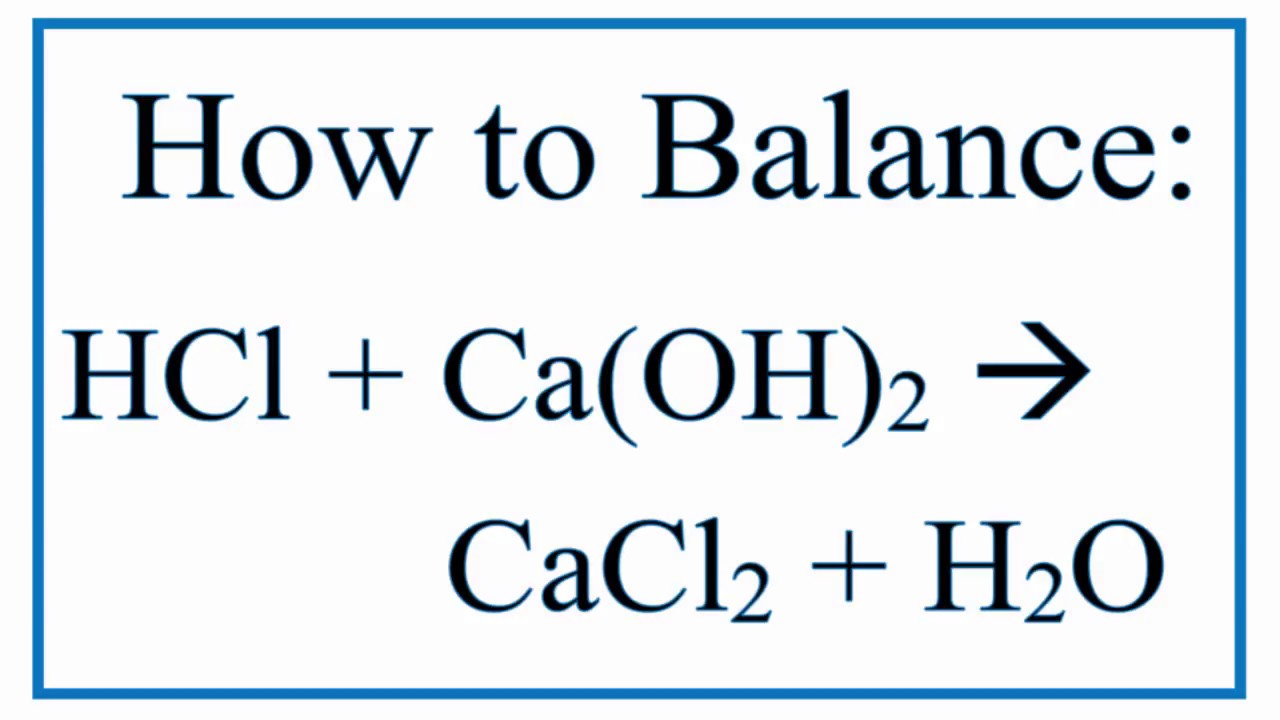
Balance Hcl Ca Oh 2 Cacl2 H2o Hydrochloric Acid And Calcium Hydroxide Youtube

A Substitute Formulae For Names And Balance The Following Equation Calcium Carbonate Reacts Youtube
0 Comments